
Note that the value for the standard absolute entropy for gaseous water (188.7 J K -1 mol -1) is greater than for liquid water (69.9 J K -1 mol -1). Note that water exists in two different states at 298.15 K and atmospheric pressure, as a liquid (H 2O (l)) and as a gas (H 2O (g)). S° (diamond) = 2.38 J K -1 mol -1 (3-dimensional covalent lattice) S° (graphite) = 5.74 J K -1 mol -1 (2-dimensional covalent lattice) S° (NaCl (g)) = 72.4 J K -1 mol -1 (3-dimensional ionic lattice) Note the (generally) large positive values of S° for gaseous substances in which the molecules are chaotically and randomly distributed:Īnd the (generally) smaller positive values of S° for solid substances in which intermolecular forces act to keep in the particles in a more structured and ordered array: Some examples are given in the table below: The values of standard absolute entropy (S°) have been tabulated for many substances. The entropy of a substance reflects the energy distribution (joules, J) at a specific temperature (kelvin, K) for a specific amount of substance (moles, mol), so the units of standard absolute entropy are J K -1 mol -1. Standard absolute entropy is given the symbol S° Standard absolute entropy refers to the absolute entropy of a substance in its standard state (that is, its state at 298.15 K and atmospheric pressure).
S T is then referred to as the absolute entropy of this crystal at temperature T K. We can substitute 0 for S 0 in the equation to get: Since the entropy of a perfect crystal at 0 K is zero: Then, the increase in the entropy of the crystal when heated from 0 K to a higher temperature of T K is: If ΔS° reaction is negative (ΔS° reaction 0 K then S > 0

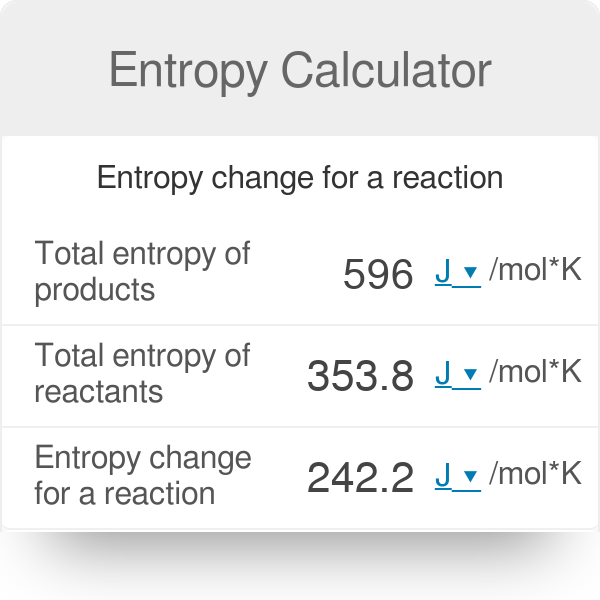
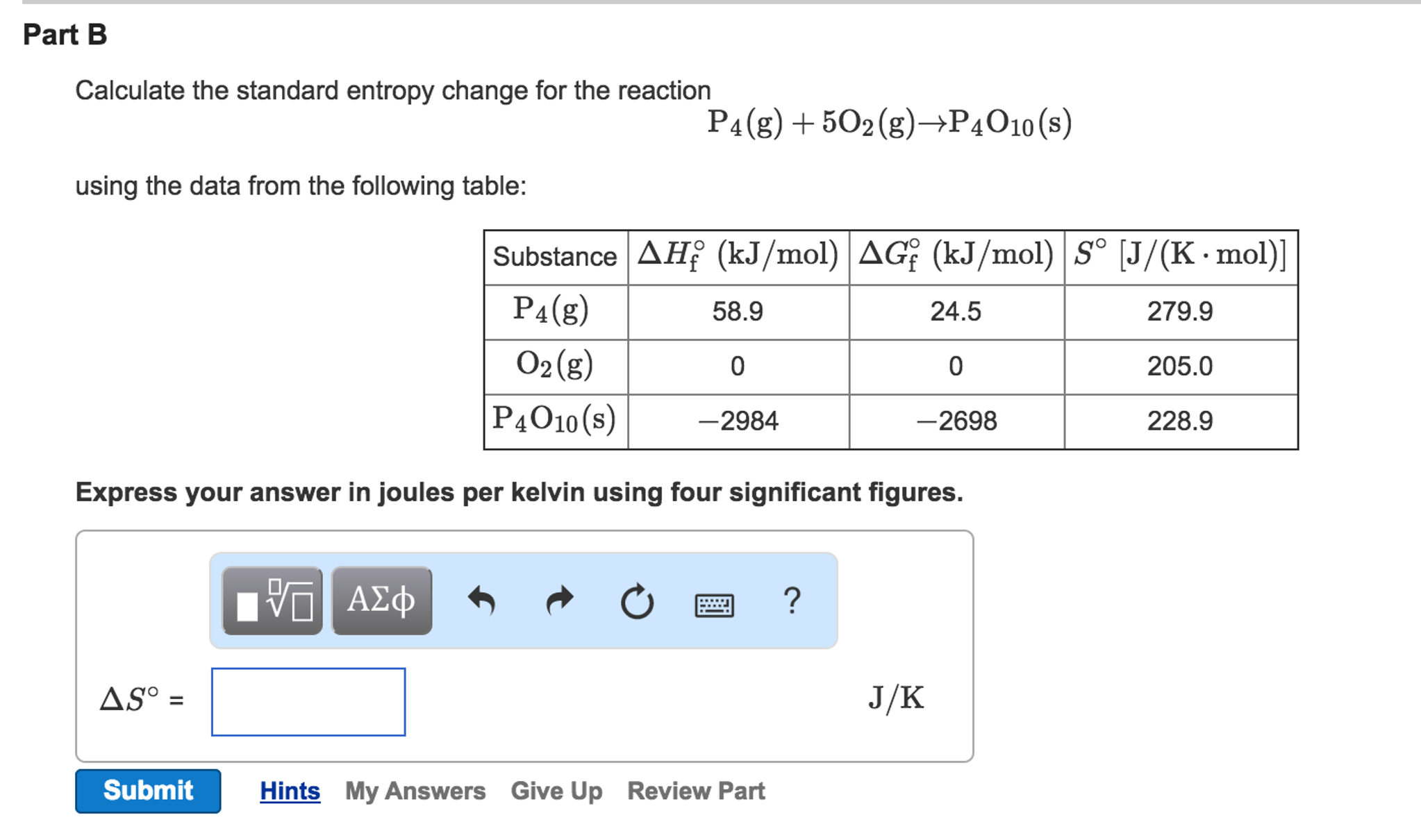
If ΔS° reaction is positive (ΔS° reaction > 0), the entropy of the system increased.For the general reaction in which reactants A and B react to produce products C and D:.ΔS° reaction = ΣS° products - ΣS° reactants

The change in standard absolute entropy for a chemical reaction (ΔS°) can be calculated using these tabulated values:.Values of standard absolute entropy (S°) for many substances have been tabulated.Standard absolute entropy values are given in units of joules per kelvin per mole J K -1 mol -1.The standard absolute entropy of a substance, S°, is the absolute entropy of a substance in its standard state (298.15 K, 100 kPa).The absolute entropy (3) of a substance, S T, is the increase in entropy when a substance is heated from 0 K to a temperature of T K.The third law of thermodynamics states that at absolute zero (0 K) (1) the entropy of a pure, perfect crystalline solid (S 0) is zero (0) (2):.You need to become an AUS-e-TUTE Member! Standard Absolute Entropy Change Calculations (ΔS°) Chemistry Tutorial Key Concepts Want chemistry games, drills, tests and more? Inlet Mass Flows equal Outlet Mass Flow.Standard Absolute Entropy Change Calculations Chemistry Tutorial More Free Tutorials Become a Member Members Log‐in Contact Us.
#Entropy change calculator generator#


 0 kommentar(er)
0 kommentar(er)
